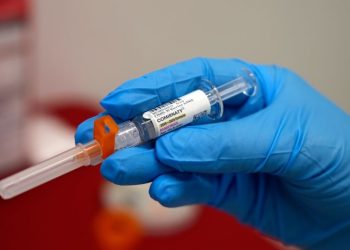
The supply of GLP-1 drugs for weight loss and diabetes treatment is expected to tighten this week with a federal deadline to halt the sale and production of off-brand products that many patients in the United States have come to rely on.
Starting in 2022, increased demand led to shortages of semaglutide injections sold by Novo Nordisk under the brand names Ozempic and Wegovy, as well as tirzepatide injections Zepbound and Mounjaro from competitor Eli Lilly. Compounding pharmacies were allowed to step in to fill supply gaps while name-brand products were in short supply, selling copycat products that used the same active ingredients but were not approved by the US Food and Drug Administration.
But the FDA declared months ago that the shortages of semaglutide and tirzepatide injections had ended, effectively ending flexibilities for compounded products. The grace period for manufacturers to stop producing and selling compounded tirzepatide ended in March, and Thursday is the final cutoff date for compounded semaglutide.
Thousands – and perhaps millions – of patients have been using these compounded GLP-1 receptor agonist medications, which are typically sold at a lower price point than the brand-name products, making them more accessible to many.
Michelle Pierce, who gets compounded semaglutide injections from Olympia Pharmaceuticals for help with weight management and high blood sugar, said her insurance denied her requests for GLP-1 medications multiple times before she explored options for compounded products. The effects have been “life–changing,” says the 25-year-old from Texas, and she’s scared to undo progress that has helped her avoid back surgery and get her blood sugar A1C level to the lowest it’s been.
“Now that it’s coming off shortage, I am planning to get off the medication. I don’t really have any other options. I absolutely cannot afford to completely pay out of pocket,” she said.
Olympia Pharmaceuticals has been providing vials of compounded GLP-1 drugs for more than 70,000 people each week, said Josh Fritzler, the company’s chief financial officer. GLP-1s accounted for about 40% of the company’s production, and it’s been planning for the stop date since the end of the shortage was announced.
“We sat down and said, ‘Here’s our goals, here’s how we’re going to process, here’s what we can do to help advocate for patient access, here’s what we can do internally to make sure we have product, and here’s our deadlines,’ ” Fritzler said. “We had to be transparent. … ‘OK, we’re going to prioritize the shortage need for the next three months to make sure that we can meet as many patients as possible before this transition is over. Because a lot of them are scared that they’re going to run out.’ ”
Dr. Jody Dushay, an endocrinologist at Beth Israel Deaconess Medical Center and assistant professor of medicine at Harvard Medical School, said she doesn’t support the use of compounded GLP-1 drugs but also worries that restrictions could add new strain to the overall supply.
“You just don’t have that security of [compounded versions] being FDA-regulated. I don’t know what’s in this compound. I don’t know about purity, I don’t know about safety. I don’t know about dosing. I don’t know about drug interactions,” Dushay said. “I really wouldn’t want to be responsible for that.”
But with the new restrictions, she said, she expects that some people who have been relying on compounded products may start to seek out new prescriptions from providers like her – and that could create a situation similar to when GLP-1 injections first became popular.
People who use GLP-1 injections typically start with low doses of the medication and gradually scale up. Compounded versions of the drugs don’t always follow the same standards for efficacy and strength as the branded products, Dushay said, making one-to-one swaps difficult – so she would assess prescribing needs like new for each patient, no matter what dose of compounded medication they had been using.
“I would start them over again. I would start them at the starting dose,” Dushay said. “So the question is: Is there going to be increased pressure on the starting doses of tirzepatide and semaglutide?”
The restrictions on compounded GLP-1 products come as concerns about existing shortages are bubbling.
The FDA declared that shortages of tirzepatide and semaglutide had ended when the drug manufacturers’ “stated product availability and manufacturing capacity can meet the present and projected national demand.”
“Patients and prescribers may still see intermittent localized supply disruptions as the products move through the supply chain from the manufacturer and distributors to local pharmacies,” the agency said when each shortage was declared to be over.
A Lilly spokesperson said in a statement that Zepbound and Mounjaro are “fully available” for patients and they “should not be exposed to untested and unapproved knockoffs.”
“Anyone continuing to sell mass-compounded tirzepatide, including by referring to it as ‘personalized,’ ‘tailored’ or something similar, is breaking the law and putting patients at risk,” Lilly’s statement said.
Both Novo Nordisk and Eli Lilly have taken steps to try to lower costs and ease access, such as creating online platforms and offering single-dose vials instead of injector pens.
“I have had direct conversations on behalf of the Outsourcing Facilities Association and provided information to the administration – both the FDA and HHS – that shows that I believe that these products – both GLP-1s – are still in shortage,” said Lee Rosebush, chairman of the trade association, which represents large-scale compounding pharmacies known as 503Bs. “What I’m afraid of happening at the end of this week when the deadline hits is that patients and providers won’t have access to the medications they need, and they will be financially impacted as they move forward because of this.”
The Outsourcing Facilities Association filed lawsuits against the FDA about the “sudden removal” of tirzepatide and semaglutide from the drug shortage list, but the judge in both cases ruled to allow the FDA plan to block compounded products to continue.
Although health care providers say they have noticed improvements in the availability of GLP-1 drugs since the shortage ended, volatile insurance policies around coverage of the drugs have hampered accessibility.
“The shortage is much better; insurance coverage is much worse,” said Dr. Disha Narang, an endocrinologist and director of obesity medicine at Endeavor Health. “From a practical standpoint, patients are unable to get employer benefits for medication, which now almost upwards of 50% of our country can potentially qualify for. So it’s a very strange time where you’re still trying to justify to insurance companies that obesity is a chronic disease.”
Pierce, who has been using a compounded product, said she has stocked up on six months of supply. But in online community forums she’s a part of, some members are just starting on the compounded drugs without knowing that they could lose access very soon.
“I am definitely afraid and will do whatever it takes to try to avoid the fallout,” she said. “But I don’t really have much control over the situation, unfortunately.”